What is the
difference between synthetic and conventional oil?
Oil, whether synthetic or petroleum-based, consists of molecular chains of
hydrogen and carbon atoms, referred to as hydrocarbons. Petroleum crude oil is a
thick, highly flammable dark-brown or greenish liquid with high energy
densities. Many contaminating elements exist in this complex mixture of
hydrocarbons, including sulfur, nitrogen, oxygen and metal components such as
nickel or vanadium. Petroleum crude oil is the raw material used for a wide
variety of petrochemicals, including solvents, fertilizers, plastics and
lubricants.
The oil refining process separates the various types of
molecules in the oil by weight, resulting in a concentrated batch suitable for
today’s uses such as gasoline, LPG, kerosene or base oils for lubricants. The
chemical composition of conventional motor oil can vary substantially and
depends on the raw crude oil refining process.
While petroleum base oils
are refined, synthetic base oils are manufactured and can achieve a higher
performance level. Synthetic oil is chemically engineered for a certain
molecular composition with a tailored and uniform structure. Such fine-tuned
control over the final molecular composition of synthetic oils is the key to the
superior performance properties of these fluids. Designing molecular structures
in a planned and orderly fashion results in molecules, and end-products, that
are far more stable than their refined petroleum counterparts.
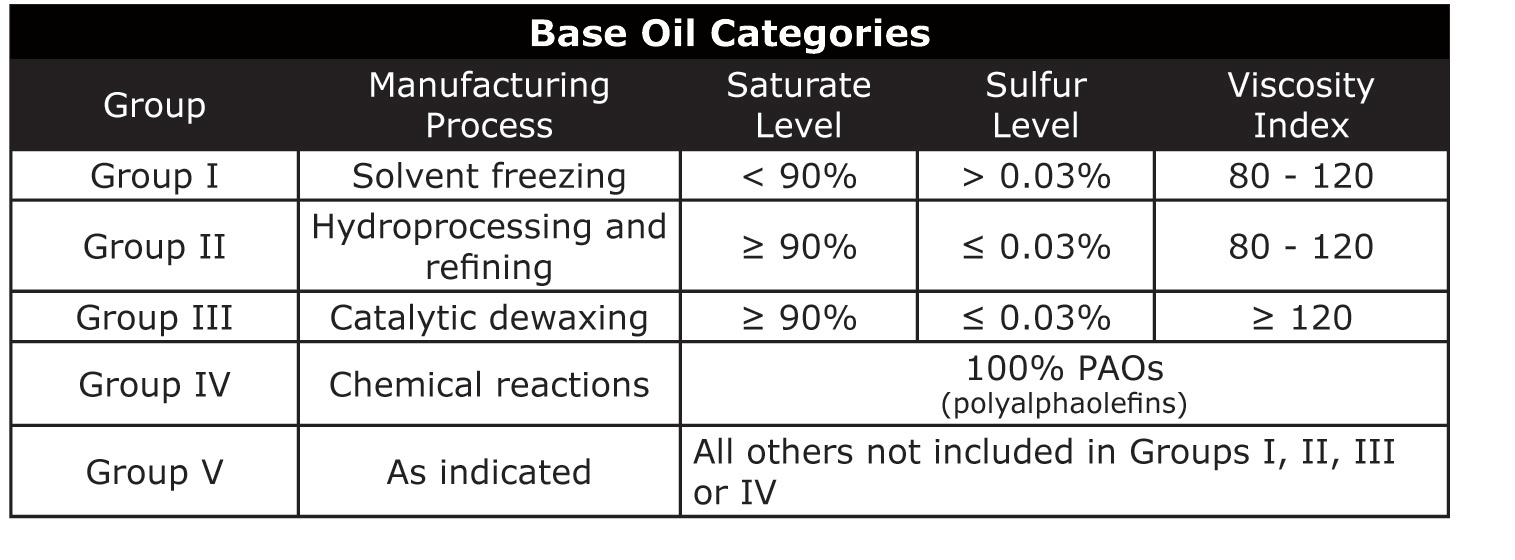
Base Oil Groups
The entire range of base oils, including conventional petroleum products, are
divided into five groups based on the level of saturates (saturated molecules),
sulfur and viscosity index. In general, the chemical composition and performance
properties of the base oil categories improve with advancing group number. For
instance, Group I has a lower concentration of saturates than Group II, while
Group II has a lower concentration of saturates than Group III base oils. Today,
Group III, Group IV and Group V base oils are considered synthetic.
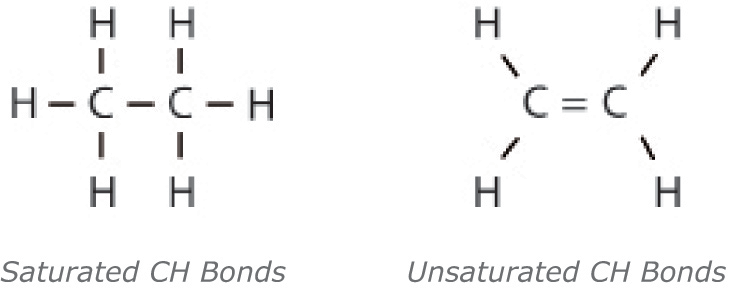
Saturated molecules contain a higher percentage of
carbon-hydrogen (CH) bonds, which limits the available sites to which other,
harmful molecules can attach. When other molecules, such as oxygen, attach to
oil molecules, they break down the molecular composition of the oil and weaken
its performance. Saturated molecules are beneficial in lubricating fluids
because they remain stable longer, resulting in a more durable lubricant.
Unsaturated molecules have fewer single carbon-hydrogen bonds and are therefore
less stable.
Sulfur is a naturally occurring, inorganic element that
readily reacts with oxygen molecules and is detrimental to oil performance.
Synthetic base oils have less sulfur than conventional base oils.
Viscosity
index refers to the temperature-viscosity relationship of lubricating fluids.
Oils with a high viscosity index (VI) are less affected by temperature; those
with low VI are affected more. Oils with a VI less than 120 (Groups I & II)
are more susceptible to viscosity variance because of temperature. The viscosity
index of synthetic base oils is higher than that of conventional petroleum base
oils.
Viscosity index refers to the temperature-viscosity relationship of
lubricating fluids. Oils with a high viscosity index (VI) are less affected by
temperature; those with low VI are affected more. Oils with a VI less than 120
(Groups I & II) are more susceptible to viscosity variance because of
temperature. The viscosity index of synthetic base oils is higher than that of
conventional petroleum base oils.
Pure, Uniform Molecules Form Strong, Stable Lubricants
Petroleum oils have molecular structures that are randomly organized and,
consequently, have limited performance abilities. Their varied and inconsistent
molecular structure results in less film strength and lubricity. Their
paraffinic wax content also makes them more susceptible to viscosity variance
and cold-temperature flow problems.
On the other hand, synthetic base oil
molecules are chemically controlled, which provides increased film strength and
lubricity over petroleum oils.
The performance qualities of base oils
have a marked impact on the performance qualities of the finished product.
Synthetic base oils provide key features and customer benefits including better
wear protection, more horsepower, increased engine cleanliness, improved fuel
economy, easier cold starts and longer oil life.
What roles do additives play in motor oil
performance?
Most lubricating oils have other chemicals added to improve the overall
performance of the fluid. Chemical additives are used to enhance the beneficial
properties of the base oil or to make up for oil deficiencies. For passenger car
motor oils, base oil makes up 70 percent to 80 percent of the final product; the
other 20 percent to 30 percent is comprised of additive
chemistry.
Additives help lubricants stand up to extreme operating
environments. Even the best base oil would not be able to protect as well
against the effects of heat, shearing forces, chemical and water dilution,
corrosion and wear particles. In short, additives make good base oils even
better. They give good base oils the performance benefits consumers have come to
expect, such as multi-grade performance, extended drain intervals and
extreme-pressure performance.
Anti-wear Agents chemically react to
form a film barrier that prevents metal-to-metal contact and
wear.
Antioxidants reduce the tendency for oil to react with
oxygen and reduce sludge buildup.
Dispersants help to suspend and
disperse contaminants in the oil to keep engine surfaces free of sludge and
deposits. They fight the build-up of corrosive acids and are most efficient at
controlling low-temperature deposits.
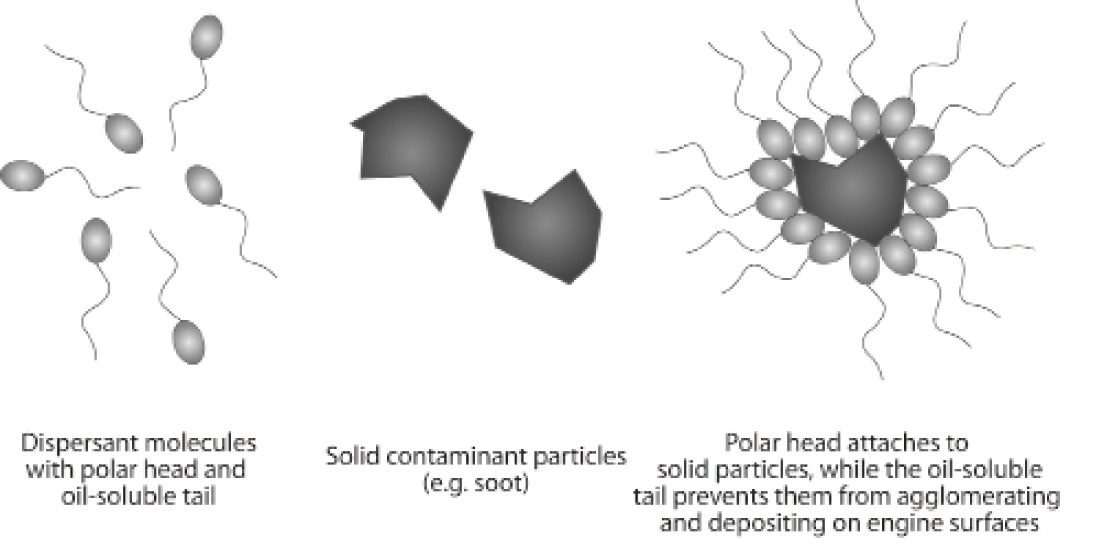
Detergents help to suspend and disperse contaminants in
the oil to keep engine surfaces free of sludge and deposits. They are most
efficient at controlling high-temperature deposits.
Extreme-Pressure
Additives coat metal surfaces to prevent close-contact components from
seizing under extreme pressure. They are activated by high temperatures and high
loads to react with the metal’s surface to form a sacrificial wear layer on
components.
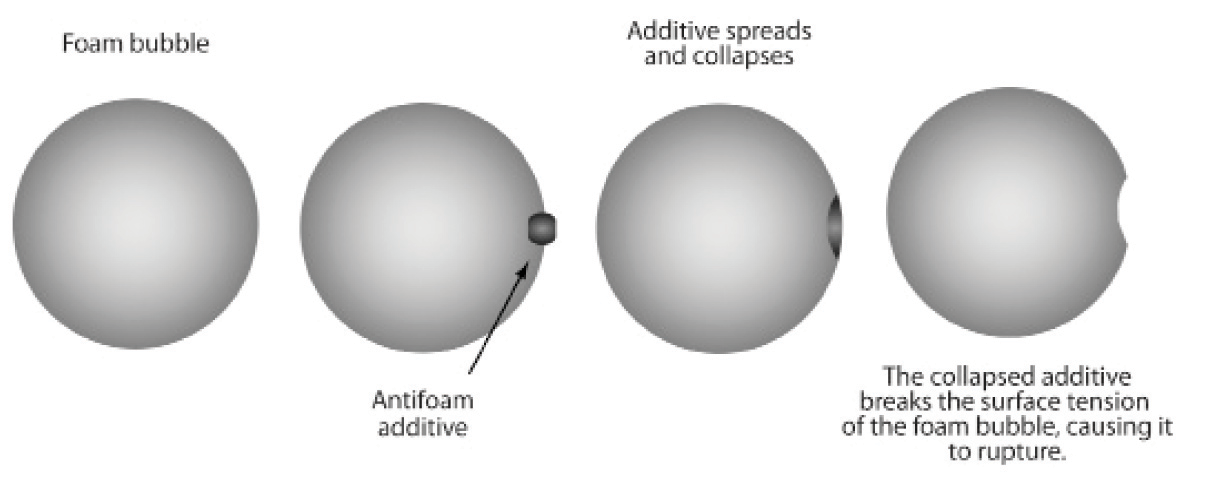
Foam Inhibitors reduce the surface tension of air
bubbles and causes them to collapse.
Friction Modifiers can be used to give oil more ‘slippery’
characteristics. In engine oils, friction modifiers are used to increase the
oil’s lubricity for the purpose of reducing friction and improving fuel
economy.
Pour Point Depressants give high-viscosity oils good
low-temperature properties. Pour point depressant polymers inhibit the formation
of crystals to minimize low-temperature viscosity increase.
Rust &
Corrosion Inhibitors form a protective barrier over component surfaces to
seal out water and contaminants. While most rust and corrosion inhibitors work
by forming a physical barrier, some rust inhibitors function by neutralizing
acids.
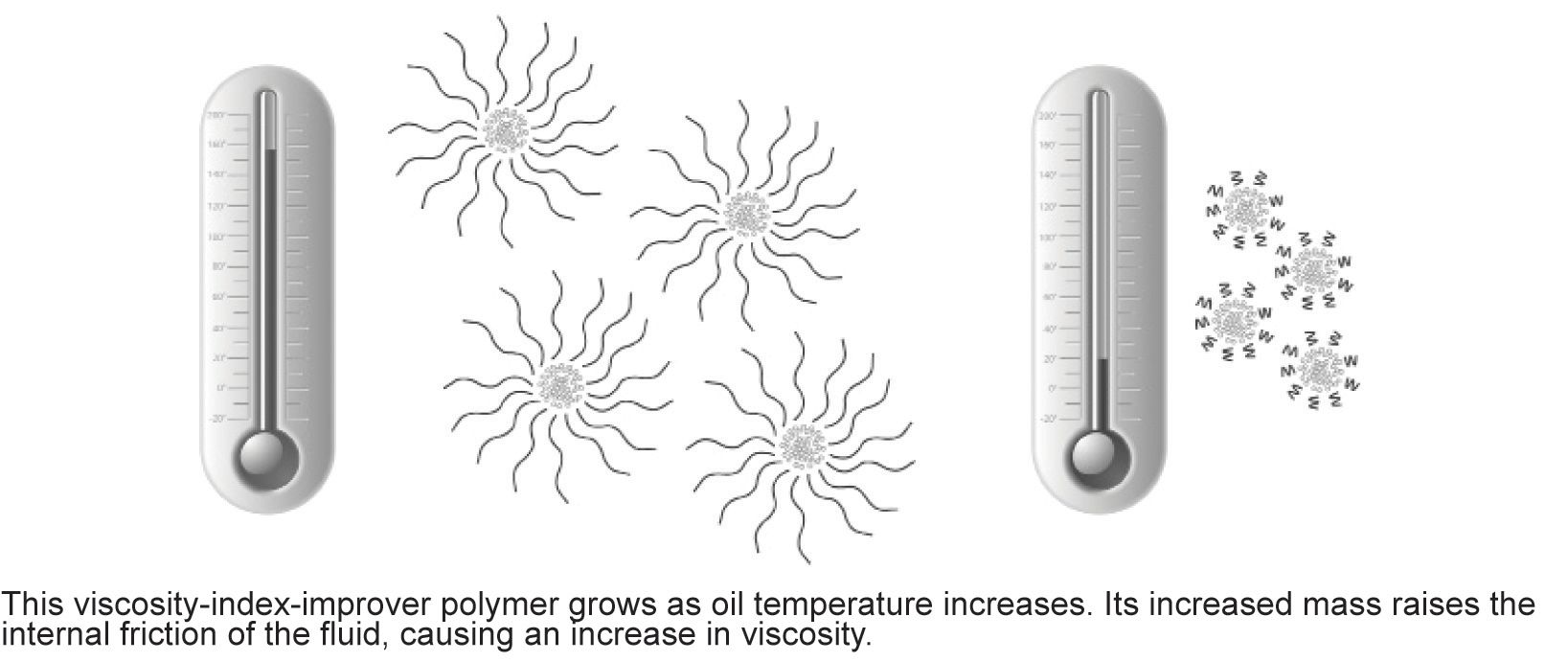
Viscosity Index Improvers are long-chain polymers that help
control the viscosity of multi-grade engine oils. They expand and contract as
temperatures vary. High temperatures cause VI improvers to expand and reduce oil
thinning; low temperatures cause the VI improvers to contract and have little
impact on oil viscosity.
 |

